 Dihedral (Shanghai) Science and Technology Co., Ltd
Dihedral (Shanghai) Science and Technology Co., Ltd
- Home
-
Products
-
- Semiconductor crystal
-
Single crystal substrate
-
Multifunctional single crystal substrate
- Barium titanate (BaTiO3)
- Strontium titanate (SrTiO3)
- Iron doped strontium titanate (Fe:SrTiO3)
- Neodymium doped strontium titanate (Nd:SrTiO3)
- Aluminium oxide (Al2O3)
- Potassium tantalum oxide (KTaO3)
- Lead magnesium niobate–lead titanate (PMN-PT)
- Magnesium oxide (MgO)
- Magnesium aluminate spinel (MgAl2O4)
- Lithium aluminate (LiAlO2)
- Lanthanu m aluminate (LaAlO3)
- Lanthanu m strontium aluminate (LaSrAlO4)
- (La,Sr)(Al,Ta)O3
- Neodymium gallate (NdGaO3)
- Terbium gallium garnet (TGG)
- Gadolinium gallium garnet (GGG)
- Sodium chloride (NaCl)
- Potassium bromide (KBr)
- Potassium chloride (KCl)
-
Multifunctional single crystal substrate
-
Functional crystal
- Optical window
- Scintillation crystal
-
Laser crystal
- Rare earth doped lithium yttrium fluoride (RE:LiYF4)
- Rare earth doped lithium lutetium fluoride (RE:LiLuF4)
- Ytterbium doped yttrium aluminium garnet (Yb:YAG)
- Neodymium doped yttrium aluminium garnet (Nd:YAG)
- Erbium doped yttrium aluminium garnet (Er:YAG)
- Holmium doped yttrium aluminium garnet (Ho:YAG)
- Nd,Yb,Er,Tm,Ho,Cr,Lu Infrared laser crystal
- N* crystal
- Metal single crystal
- Material testing analysis
- Material processing
- Scientific research equipment
-
-
Epitaxial Wafer/Films
-
Inorganic epitaxial wafer/film
- Gallium Oxide epitaxial wafer (Ga2O3)
- ε - Gallium Oxide (Ga2O3)
- Platinum/Titanium/Silicon Dioxide/Silicon epitacial wafer (Pt/Ti/SiO2/Si)
- Lithium niobate thin film epitaxial wafer
- Lithium tantalate thin film epitaxial wafer
- InGaAs epitaxial wafer
- Gallium Nitride(GaN) epitaxial wafer
- Epitaxial silicon wafer
- Yttrium Iron Garnet(YIG) epitaxial wafers
- Fullerenes&Fullerols
-
Inorganic epitaxial wafer/film
- Functional Glass
- Fine Ceramics
-
2-D material
- 2-D crystal
-
Layered transition metal compound
- Iron chloride (FeCl2)
- Niobium sulfide (NbS3)
- Gallium telluride iodide (GaTeI)
- Indium selenide (InSe)
- Copper indium phosphide sulfide (CuInP2S6)
- Tungsten sulfide selenide (WSSe)
- Iron germanium telluride (Fe3GeTe2)
- Nickel iodide (NiI2)
- Iron phosphorus sulfide (FePS3)
- Manganese phosphorus selenide (MnPSe3)
- Manganese phosphorus sulfide (MnPS3)
- Interface thermal conductive materials
-
Epitaxial Wafer/Films
-
-
High-purity element
- Non-metallic
-
Metal
- Scandium (Sc)
- Titanium (Ti)
- Indium (In)
- Gallium (Ga)
- Bismuth (Bi)
- Tin (Sn)
- Zinc (Zn)
- Cadmium (Cd)
- Antimony (Sb)
- Copper (Cu)
- Nickel (Ni)
- Molybdenum (Mo)
- Aluminium (Al)
- Rhenium (Re)
- Hafnium (Hf)
- Vanadium (V)
- Chromium (Cr)
- Iron (Fe)
- Cobalt (Co)
- Zirconium (Zr)
- Niobium (Nb)
- Tungsten (W)
- Germanium (Ge)
- Iron(Fe)
-
Compound raw materials
-
Oxide
- Tungsten Trioxide (WO3)
- Hafnium Dioxide (HfO2)
- Ytterbium Oxide (Yb2O3)
- Erbium Oxide (Er2O3)
- Lanthanu m Oxide (La2O3)
- Cerium Dioxide (CeO2)
- Tin Dioxide (SnO2)
- Niobium Oxide (Nb2O3)
- Zirconium Dioxide (ZrO2)
- Zinc Oxide (ZnO)
- Copper Oxide (CuO)
- Magnetite (Fe3O4)
- Titanium Dioxide (TiO2)
- Samarium (III) oxide (Sm2O3)
- Silicon Dioxide (SiO2)
- Aluminum Oxide (Al2O3)
- Gallium Oxide Ga2O3(Powder)
- Sulfide
- Fluoride
- Nitride
- Carbide
-
Halide
- Gallium Chloride (GaCl3)
- Indium Chloride (InCl3)
- Aluminum Chloride (AlCl3)
- Bismuth Chloride (BiCl3)
- Cadmium Chloride (CdCl2)
- Chromium Chloride (CrCl2)
- Chromium Chloride Hydrate (CrCl2(H2O)n)
- Copper Chloride (CuCl)
- Copper Chloride II (CuCl2)
- Cesium Chloride (CsCl)
- Europium Chloride (EuCl3)
- Europium Chloride Hydrate (EuCl3.xH2O)
- Magnesium Chloride (MgCl2)
- Sodium Chloride (NaCl)
- Nickel Chloride (NiCl2)
- Indium Chloride (InCl3)
- Indium Nitrate Hydrate (In(NO3).xH2O)
- Rubidium Chloride (RbCl3)
- Antimony Chloride (SbCl3)
- Samarium Chloride (SmCl3)
- Samarium Chloride Hydrate (SmCl3.xH2O)
- Scandium Chloride (ScCl3)
- Tellurium Chloride (TeCl3)
- Tantalum Chloride (TaCl5)
- Tungsten Chloride (WCl6)
- Aluminum Bromide (AlBr3)
- Barium Bromide (BaBr2)
- Cobalt Bromide (CoBr2)
- Cadmium Bromide (CdBr2)
- Gallium Bromide (GaBr3)
- Gallium Bromide Hydrate (GaBr3.xH2O)
- Nickel Bromide (NiBr2)
- Potassium Bromide (KBr)
- Lead Bromide (PbBr2)
- Zirconium Bromide (ZrBr2)
- Bismuth Bromide (BiBr4)
- Bismuth Iodide (BiI3)
- Calcium Iodide (CaI2)
- Gadolinium Iodide (GdI2)
- Cobalt Iodide (CoI2)
- Cesium Iodide (CsI)
- Europium Iodide (EuI2)
- Lithium Iodide (LiI)
- Lithium Iodide Hydrate (LiI.xH2O)
- Gallium Iodide (GaI3)
- Gadolinium Iodide (GdI3)
- Indium Iodide (InI3)
- Potassium Iodide (KI)
- Lanthanu m Iodide (LaI3)
- Lutetium Iodide (LuI3)
- Magnesium Iodide (MgI2)
- Sodium Iodide (NaI)
-
Oxide
-
High-purity element
-
-
Sputtering Target
-
Metal target material
- Gold (Au(T))
- Silver (Ag(T))
- Platinum (Pt(T))
- Palladium (Pd(T))
- Ruthenium (Ru(T))
- Iridium (Ir(T))
- Aluminium (Al(T))
- Copper (Cu(T))
- Titanium (Ti(T))
- Nickel (Ni(T))
- Chromium (Cr(T))
- Cobalt (Co(T))
- Iron (Fe(T))
- Manganese (Mn(T))
- Zinc (Zn(T))
- Vanadium (V(T))
- Tungsten (W(T))
- Hafnium (Hf(T))
- Niobium (Nb(T))
- Molybdenum (Mo(T))
- Lanthanu m (La (T))
- Cerium (Ce (T))
- Praseodymium (Pr (T))
- Neodymium (Nd (T))
- Samarium (Sm (T))
- Europium (Eu (T))
- Gadolinium (Gd (T))
- Terbium (Tb (T))
- Dysprosium (Dy (T))
- Holmium (Ho (T))
- Erbium (Er (T))
- Thulium (Tm (T))
- Ytterbium (Yb (T))
- Lutetium (Lu (T))
- Alloy target material
- Semiconductor target material
-
Oxide target material
- Aluminum Oxide (Al2O3(T))
- Silicon Dioxide (SiO2(T))
- Titanium Dioxide (TiO2(T))
- Chromium Oxide (Cr2O3(T))
- Nickel Oxide (NiO(T))
- Copper Oxide (CuO(T))
- Zinc Oxide (ZnO(T))
- Zirconium Oxide (ZrO2(T))
- Indium Tin Oxide (ITO(T))
- Indium Zinc Oxide (IZO(T))
- Aluminum Doped Zinc Oxide (AZO(T))
- Cerium Oxide (CeO2(T))
- Tungsten Trioxide (WO3(T))
- Hafnium Oxide (HfO2(T))
- Indium Gallium Zinc Oxide (IGZO(T))
- Nitride target material
- Sulfide target material
-
Antimony tellurium selenium boron target material
- Magnesium Boride (MgB2(T))
- Lanthanu m Hexaboride (LaB6(T))
- Titanium Diboride (TiB2(T))
- Zinc Selenide (ZnSe(T))
- Zinc Antimonide (Zn4Sb3(T))
- Cadmium Selenide (CdSe(T))
- Indium Telluride (In2Te3(T))
- Tin Selenide (SnSe(T))
- Germanium Antimonide (GeSb(T))
- Antimony Selenide (Sb2Se3(T))
- Antimony Telluride (Sb2Te3(T))
- Bismuth Telluride (Bi2Te3(T))
-
Metal target material
-
Sputtering Target
-
- Services
- Media
- Partner
- Contact Us
- About
- Home
- Products
- Epitaxial Wafer/Films
- Inorganic epitaxial wafer/film
- Fullerenes&Fullerols
Fullerenes&Fullerols
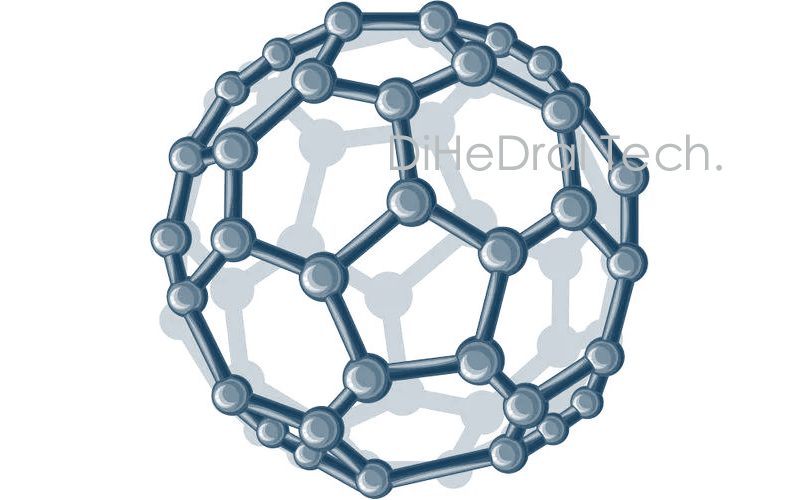
The molecular structure of C60 is a spherical 32hedron, consisting of 60 carbon atoms connected by 20 hexagonal and 12 pentagonal rings, forming a spherical hollow symmetric molecule with 30 carbon carbon double bonds. Therefore, fullerene is also known as football. Fullerene C60 has a standard spherical molecular structure, which gives it special stability.
Fullerene, due to its unique structure, has excellent properties in conductivity, thermodynamics, optics, magnetism, and chemical properties. It is one of the most important carbon containing nanomaterials in recent years. Fullerene and its derivatives have been widely used in medical, industrial, scientific research, and other fields.
Dihedral technology manufacturers provide high-quality fullerene and hydroxylated fullerene materials to meet your scientific research and product innovation needs.
Applications
>Special lubricants;
>Ultra high hardness grinding materials;
>Applications in optical computing, optical memory, optical signal processing and control, etc;
>Inhibiting chemical toxicity, resisting radiation, preventing UV damage, protecting against heavy metal cell damage, resisting cell oxidation, and absorbing free radicals can protect cells from various damages;
>Many fields include magnetic resonance imaging, anti HIV drugs, anti-cancer drugs, chemotherapy drugs, cosmetic additives, and scientific research.
Features
At the molecular level, a single C60 molecule is exceptionally hard;
The special shape of C60 molecules and their strong ability to resist external pressure;
C60 is used for detecting TNT in sensors;
The three-dimensional highly non localized electron conjugated structure in C60 molecules endows it with excellent optical and nonlinear optical properties.
-
Powder series
Product Name: Fullerene C60 Product English Name: Fullerene C60
CAS number: 99685-96-8 Product code: FC60AS1
Product purity specifications: □ 99% □ 99.5% □ 99.9%
Possible impurities: C60 oxide, C70 and its oxides, high fullerene and its oxides
Properties
Items
Results
Items
Results
Items
Results
Appearance
Brown or black powder (crystal)
Bulk modulus of elasticity
14 Gpa
Debye temperature
185 K
C-C average bond length
1.44 A
Phase transition temperature
255K,90K
Thermal conductivity(300K)
0.4 W/mk
Lattice constant
14.17 A
atom binding energy
7.4 eV
Average free path of photons
50 Á
Cso average diameter
6.83 A
Electron binding energy
2.65 eV
Dielectric constant
4.0-4.5
Co ball outer diameter
10.18 A
First ionization point position
7.58 eV
standard heat of formation
9.08 k cal mol-l
Cso ball innter diameter
3.48 A
Second ionization point position
11.5 eV
Refractive index
2.2 (600 nm)
Mass density
1.72 g/cm³
Thermal expansion coefficient
6.2×10-/cm³/K
Boiling point
800K下升华
Molecular density
1.44×10²¹/cm²
Band energy(HOMO - LUMO)
1.7 eV
Resistivity
1014 ohms m'¹
Solubility: Can dissolve in small amounts in organic solvents such as 1,2,4-trichlorobenzene, carbon disulfide, toluene, benzene, chloroform, carbon tetrachloride, n-hexane, tetrahydrofuran, acetonitrile, etc. It is insoluble in water.
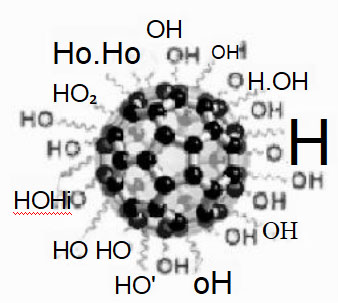
Fullerenol
Product Name: Hydroxylated Fullerene (Fullerenols) Product English Name: Hydroxylated Fullerene (Fullerenols)
Product code: HFC60AS3 Molecular formula: C60 (OH) n: mH ₂ O Number of hydroxyl groups: 24-28
Properties
Appearance and shape: Dark brown or black powder
Melting point/freezing point: Melting point/melting range:>280 ℃
Water insoluble substance,%: ≤ 0.003
Relative density: approximately 1.55 g/cm ³ At 20 ℃
Odor: No odor
Sodium (Na),%: ≤ 0.03
PH (0.1g/L, 25 ℃): 7-7.5
Gen. (in Pb),%: ≤ 0.0005
Solubility: easily soluble in water, slightly soluble in DMF, insoluble in organic solvents such as benzene, toluene, chloroform, n-hexane, acetonitrile, etc. 
Fullerol has similar chemical properties to fullerene. Introducing hydroxyl groups on fullerene improves its water solubility. Fullerol is insoluble in acetone and methanol, but soluble in DMF.
Fullerol can strongly absorb free radicals, inhibit chemical toxicity, resist radiation, UV damage, protect against heavy metal cell damage, resist cell oxidation, resist bacterial infection, and absorb free radicals, thus protecting cells from various damages.
It can be widely used in many fields such as magnetic resonance imaging, anti HIV drugs, anti-cancer drugs, chemotherapy drugs, cosmetic additives, and scientific research.

